BCL2 is a key pro-survival protein that is overexpressed in many cancers. The first generation BCL2 inhibitor venetoclax has proved highly effective in multiple hematological malignancies including CLL and AML. However, the pharmacokinetic half-life of venetoclax is 26 hours which prevents optimization of the therapeutic window. Additionally, venetoclax metabolism is influenced by Cyp3A/4 inhibitors making their use in diseases where anti-fungal prophylaxis is required challenging. Venetoclax treatment can also result in significant immunosuppression including dose-limiting neutropenia and thrombocytopenia, and the loss of multiple lymphocyte populations, due in part to its effect on BCL-xL. Herein we present the pre-clinical evaluation of a novel highly selective BCL2 inhibitor, ZE50-0134, with equivalent anti-tumor efficacy, improved safety profile, and limited impact on non-malignant immune populations.
ZE50-0134 binds the P2 pocket of BCL2, thereby increasing selectivity toward BCL-2, ZE50-0134 showed a 4600-fold greater selectivity for BCL2 over BCL-xL compared to venetoclax with only an 84-fold greater selectivity. In vivo murine and canine pharmacokinetics showed that ZE50-0134 has a substantially shorter half-life than venetoclax suggesting feasibility for periodic pulse dosing to limit the on-target adverse effects of BCL2 inhibition. The ADME properties of ZE50-0134 were exceptional. An in vivo murine study examining the pharmacology of ZE50-0134 or venetoclax given with ketoconazole, a strong CYP3A inhibitor, was performed demonstrating a 0.84-fold decrease in AUC with ZE50-0134 vs. a 1.52-fold increase in AUC with venetoclax suggesting less Cyp3A influence on ZE50-0134. Toxicology in rats and dogs suggest a 19-fold margin between therapeutically effective dose and toxicity with ZE50-0134.
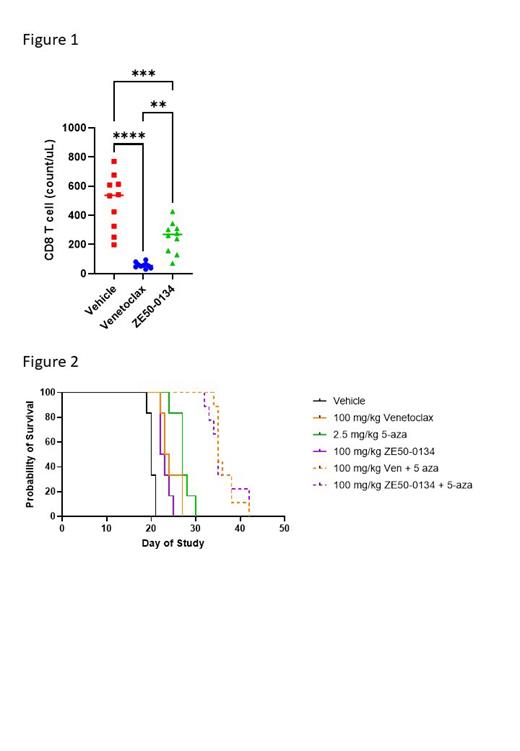
Given this data, we sought to validate that ZE50-0134 has less impact on normal immune populations. Wild-type C57BL6/J mice were treated daily with 100 mg/kg ZE50-0134, 100 mg/kg venetoclax or vehicle. A comprehensive 36-color spectral flow cytometry immune profiling panel was used to evaluate the effect of BCL2 inhibitors on multiple immune populations including B, T and NK cells as well as monocyte/macrophage and dendritic cell subsets in the peripheral blood. Venetoclax treated mice demonstrated a significant loss of multiple lymphocyte populations, including B, T, and NK cells, in the peripheral blood. Particularly, from Day 14 venetoclax treatment significantly reduced the percentage and absolute count of CD8 T cells (Figure 1). Some reduction in CD8 T cells was also observed with ZE50-0134 treatment, however it was significantly less than with venetoclax. No changes in the frequency of any normal myeloid population were observed.
We next sought to examine the effectiveness of ZE50-0134 in B-cell and myeloid malignancies. Primary CLL cells were treated for 4 and 24 hours, corresponding to the expected half-life of ZE50-0134 and venetoclax, followed by washout. Cytotoxicity at 72 hours for both ZE50-0134 and venetoclax were equivalent for both short and prolonged treatment. We then assessed ZE50-0134 and venetoclax in vivo at 100 mg/kg in the RSV4;11 subcutaneous lymphoid model where we observed similar tumor growth regression. In AML, venetoclax is typically not effective as monotherapy and if often used in combination with azacytidine. To assess the relative efficacy of venetoclax and ZE50-0134 in combination with Azacitidine (5-Aza), we used the MOLM-13 cell line disseminated xenograft model. NCG mice were engrafted with MOLM-13 cells via the tail vein. From day 3, mice were treated with daily oral gavage of venetoclax or ZE50-0134 (100 mg/kg) alone or in combination with weekly intravenous 5-Aza (2.5 mg/kg). ZE50-0134 and venetoclax performed equally as single agents (median survival 23.5 days vs 22.5 days) and in combination with 5-Aza (median survival 35 days vs 35 days) (Figure 2).
Together, this data suggests that ZE50-0134 is a highly effective BCL2 inhibitor with substantially less impact on the normal immune cells responsible for both anti-tumor and anti-infectious immunity. The pharmacology of this agent makes it an ideal therapeutic to optimize tumor BCL2 dependence and avoid untoward toxicity that occurs through continuous BCL2 targeting. This data supports the further clinical development of ZE50-0134 in both B cell malignancies and AML.
Disclosures
Orry:MolSoft LLC: Current Employment. Lam:MolSoft LLC: Current Employment. Abagyan:MolSoft LLC: Current Employment. Kysil:ChemDiv Inc: Current Employment. Gukasyan:ChemDiv Inc: Current Employment. Mitkin:ChemDiv Inc: Current Employment. Karapetian:ChemDiv Inc: Current Employment. Ryakhovskiy:ChemDiv Inc: Current Employment. Bulanova:ChemDiv Inc: Current Employment. Parchinsky:ChemDiv Inc: Current Employment. Ivachtchenko:ChemDiv Inc: Current Employment. Dokukina:Eilean Theraputics: Current Employment, Current equity holder in private company. Pushechnikov:ChemDiv Inc: Current Employment. Savchuk:Eilean Therapeutics: Current Employment, Current equity holder in private company. Dukes:Eilean Therapeutics: Current Employment, Current equity holder in private company. Burd:Eilean Theraputics: Current Employment, Current equity holder in private company. Byrd:Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES; OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding.